SCI论文(www.lunwensci.com):
摘要:肝纤维化是一种以细胞外基质沉积为特点的肝内慢性损伤修复过程,是各种慢性肝病的共同通路。肝星状细胞(HSC)活化、增殖等是肝纤维化发生、发展的关键环节。近年研究结果显示,纤维化过程中多种miRNAs异常表达,并通过对多种信号通路的调控,参与HSC活化、增殖和凋亡过程,介导肝纤维化的发生发展。探讨miRNAs在肝纤维化的作用机制,将为肝纤维化的诊断、治疗和预后提供新思路。本文就微RNA(miRNAs)在肝纤维化中的作用及其在肝纤维化中的相关信号通路关联进行综述。
关键词:肝纤维化;微RNA;信号通路;综述
本文引用格式:申秋艳,罗伟生.miRNAs在肝纤维化中的研究进展[J].世界最新医学信息文摘,2019,19(80):64-66.
Research Progress of miRNAs in Hepatic Fibrosis
SHEN Qiu-Yan,LUO Wei-Sheng*
(Guangxi University of Traditional Chinese Medicine,Nanning Guangxi)
ABSTRACT:Hepatic fibrosis is a kind of chronic injury repair process in liver characterized by extracellular matrix deposition,which is a common pathway of various chronic hepatic diseases.Activation and proliferation of hepatic stellate cells(HSC)are key links in the occurrence and development of hepatic fibrosis.Recent studies have shown that multiple miRNAs are abnormally expressed in the course of fibrosis and are involved in the activation,proliferation and apoptosis of HSC through the regulation of various signaling pathways,mediating the occurrence and development of hepatic fibrosis.To explore the mechanism of miRNAs in hepatic fibrosis will provide new ideas for the diagnosis,treatment and prognosis of hepatic fibrosis.In this paper,the role of miRNAs in hepatic fibrosis and their correlation with signal pathways in hepatic fibrosis were reviewed.
KEY WORDS:Hepatic fibrosis;miRNA;Signal pathways;Review
0引言
肝纤维化(hepatic fibrosis,HF)是各种损伤因子作用下导致的肝内纤维结缔组织异常增生的病理过程,是肝硬化的前期病变。其中,肝星状细胞(Hepatic Stellate Cell,HSC)活化转化为肌成纤维细胞是ECM的主要来源,是肝纤维化的起始和进展中的关键步骤。现代研究发现,在肝纤维化过程中多种微RNA(microRNAs,miRNAs)异常表达,miRNAs可参与多种信号通路的调控,介导HSC的活化、增殖和凋亡,参与肝纤维化的发生发展过程。miRNAs被认为是肝星状细胞(HSCs)活化的关键调控因子。本文将集中讨论miRNAs在肝纤维化中的表达和已知的相关信号关联。
1miRNA概述
过去,人们认为只有编码蛋白的基因才具有生物功能。人类基因组计划结束后,人们发现占人类基因组95%的非编码RNA(noncoding RNAs,ncRNAs)在基因或蛋白的表达中充当着重要的调控角色,影响细胞分化凋亡、生物发育、疾病发生等。miRNA是一种短(19~25bp)的非编码短单链RNA,是众多ncRNAs的一种。miRNA通过与靶信使核糖核酸(mRNA)特异结合,从而介导转录后基因沉默,属于一个高度保守的转录后调控基因家族,在调控细胞增殖、分化、炎症、免疫等过程中起着至关重要的作用[1]。
2miRNA参与的肝纤维化信号关联
2.1miRNA调控TGF-β/Smads通路
转化生长因子-β(TGF-β)是肝脏疾病中主要的促纤维化细胞因子,TGF-β信号通路受兴奋性因子(Smad2和Smad3)和抑制性因子(Smad 7)调节。大量文献报道,miRNA可以通过调控TGF-β信号通路的调节因子介导肝纤维化过程。现代研究发现,miR-212-3p过表达可靶向下调TGF-β通路的抑制性因子Smad 7,进而促进TGF-β信号通路和HSC的活化,诱导HSC的活化标志物,如α-平滑肌肌动蛋白(α-SMA)和胶原蛋白的生成[2]。miR-130a-3p在HSCs中的过表达时,Smad2、Smad3、转化生长因子-β受体
1(TGFBR1)、TGFBR2、基质金属蛋白酶-2(MMP-2)、MMP-9、I型胶原蛋白(Col-1)和Col-4的表达下降,表明miR-130a-3p可能通过TGF-β/Smad2和Smad3信号通路,在纤维化进展中负调节HSC活化和增殖[3]。miR-17-5p可通过还原Smad7促进HSC增殖和活化[4]。miRNA-122可以通过抑制TGF-β1/Smad4信号通路,抑制由TGF-β1刺激的HSC的活化和上皮-间质转化(EMT)[5]。过表达的热休克因子1(HSF1)可促进热休克蛋白47(Hsp47)表达,导致TGF-β/Smad4信号传导途径的活化,研究报道,miR-455-3p的过表达可抑制HSF1表达并减少纤维化标志物表达[6]。miR-134还通过直接结合TGF-β-活化的激酶1-结合蛋白1(TAB1)的3'非翻译区抑制其表达,进而而抑制HSC的活化[7]。
2.2miRNA调控Hedgehog(Hh)通路
Hh信号通路与HSC的静息状态的维持密切相关。Hh的过度或持续激活可促使HSC由静息状态转变为活化状态,促进肝纤维化发生。研究发现,升高的miRNA-214通过抑制Hh通路的负调节因子Sufu的表达,促进HSC活化,促进纤维细胞外基质的积累和促纤维化基因的表达[8]。miR-9可通过靶向抑制多耐药相关蛋1(MRP1)的表达,进而抑制Hh通路对HSCs的活化和增殖,在纤维化肝组织和活化的HSC中,miR-9水平下调[9]。
2.3miRNA调控Wnt/β-catenin通路
大量研究已证实,Wnt/β-catenin通路的异常激活加速了肝纤维化的发展。miR-17-5p通过抑制Wnt抑制因子1(WIF1)的表达激活Wnt/β-catenin途径以导致HSC活化[10]。过表达的miR-378a-3p靶向调节Wnt/β-catenin途径的成员Wnt10可导致Wnt/β-catenin途径的失活从而抑制肝纤维化,miR-145也可通过靶向调节锌指E-盒结合同源框2(ZEB2)抑制Wnt/β-catenin通路抑制肝纤维化,而研究发现肝纤维化过程中miR-378a-3p及miR-145表达降低[11,12]。此外,研究还发现,ZEB2与p53启动子的E-box结合,可抑制细胞周期因子p53的表达,从而抑制活化的HSCs衰老,促进肝纤维化[13]。
2.4miRNA调控核因子κB(nuclear factor kappa-B,NF-κB)信号通路NF-κB是广泛存在于细胞中的核转录因子,活化状态下可诱导一系列炎性因子及抗凋亡基因的表达,促进肝纤维化。研究证实,miRNA-146a-5p可通过靶向抑制肠源性内毒素(LPS)/NF-κB/Bambi信号传导通路的IL-1受体相关激酶1(IRAK1)和TNF受体相关因子-6(TRAF6),间接抑制TGF-β/Smad信号通路,阻断肝纤维化进程[14]。miR-378a-3p受Smo依赖性NF-κB信号传导调节,通过直接靶向GLI家族锌指蛋白3(Gli3),减少的Gli3和促纤维化基因的表达,抑制HSC的活化[15]。AMPK信号通过增加sirtuin 1的脱乙酰酶活性负调节NF-κB-TNFα炎症轴,在肝脏炎症和纤维化的发展过程中,MIR-378直接靶向作用于编码AMP激活的蛋白激酶γ2(AMPKγ2)的Prkag2,降低sirtuin 1活性并促进涉及NF-κB-TNFα的炎症途径[16]。
2.5miRNA调控PI3K/AKT通路
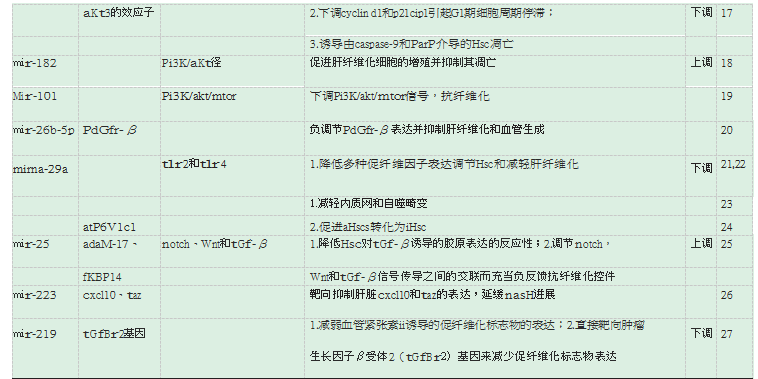
大量的文献表明,PI3K/AKT通路在促进肝纤维化中的HSCs增殖,抑制HSCs凋亡充当着重要的角色。研究报道miR-29b在活化的HSC(LX-1,HSC-T6)中的异位表达可抑制细胞活力和集落形成,并通过下调细胞周期蛋白D1(cyclin D1)和p21cip1引起G1期细胞周期停滞;此外,miR-29b还可诱导由caspase-9和PARP介导的HSC凋亡;miR-29b通过直接靶向PIK3R1和AKT3的下游效应子的3'UTR区域抑制其效应,进而抑制PI3K/AKT通路,实现抗肝纤维化[17]。miR-182在肝纤维化中高表达,并且与转录因子FOXO1表达负相关,高表达的miR-182和低表达的FOXO1通过反馈PI3K/AKT信号通路促进肝纤维化细胞的增殖并抑制其凋亡[18]。MiR-101可通过下调PI3K/Akt/mTOR信号通路实现抗纤维化[19]。
2.6miRNA调控PDGFR/MAPK通路
血小板衍生的生长因子受体-β(PDGFR-β)的表达增加,与纤维化和血管生成标志物呈正相关。纤维化肝脏中miR-26b-5p的肝脏表达降低,与PDGFR-β和纤维化以及血管生成标志物呈负相关。体内miR-26b-5p负调节PDGFR-β表达并减弱肝纤维化和血管生成。miR-26b-5p通过直接靶向PDGFR-β并与长的非编码RNA(lncRNA)母系表达的基因3(lncMEG3)相互作用抑制肝纤维发生和血管生成,这可能是肝纤维化的有效治疗策略[20]。
2.7miRNA调控TLR2、TLR4通路
Toll样受体2(TLR2)和TLR4是细菌脂蛋白和脂多糖的识别受体,在启动免疫应答、介导炎症反应、传导信号等方面发挥重要作用。在肝脏中,TLR参与肝细胞损伤的炎症反应。小鼠实验证实,miRNA-29a过表达可抑制TLR2和TLR4信号通路,降低各种促炎细胞因子及HSC的活化标志物的表达,如miR-29a表达增强可致肝组织内α-SMA、TGF-β、IL-6、IL-1β、p-Smad3、PI3K、DNA甲基转移酶3b(DNMT3b)等的表达下调,证明miRNA-29a是调节HSC和减轻肝纤维化的关键调节因子[21-22]。小鼠实验还发现,MiRNA-29a还可减轻内质网和自噬畸变抵消阻塞性黄疸诱导的纤维化[23]。在纤维化消退期间,MiRNA-29a在将活化的肝星状细胞(aHSCs)转化为灭活细胞(iHSC)中起重要作用,部分是通过调节ATP酶H+转运V1亚基C1(ATP6V1C1)实现的[24]。
2.8miRNA调控的其他靶点
miR还可同时参与多重信号通路介导肝纤维化过程,Genz,B[25]发现Notch信号传导激活剂ADAM-17和FKBP14是HSC中的miR-25靶标,在HSC活化过程中miR-25通过降低HSC对TGF-β诱导的胶原表达的反应性和调节Notch,Wnt和TGF-β信号传导之间的交联而充当负反馈抗纤维化控件。He,Y[26]报道miR-223可以靶向抑制肝脏(CXC基序)趋化因子10(Cxcl10)和具有PDZ结合基序(Taz)的表达。所周知,Cxcl10和Taz是两种促进非酒精性脂肪性肝炎(NASH)进展的因子。Ma,L等[27]研究发现,miR-219有强烈抑制肝纤维化作用,miR-219在患者血清中的表达显着降低,且表达与临床分期呈负相关,研究结果表明,miR-219可减弱血管紧张素II诱导的促纤维化标志物的表达,或直接通过靶向肿瘤生长因子β受体2(TGFBR2)基因来减少促纤维化标志物表达。
3问题与展望
近年来大量的研究表明,在肝纤维化形成过程中,miRNAs发挥着重要的作用,其中一些已被确定为潜在的诊断或治疗靶点。Nan,Y等[28]研究结果表明miR-1273g-3p水平可作为一种新型非侵入性试验用于CHC患者的诊断与肝纤维化分期预测。Feng,MH等[29]证明萝卜硫素(SFN)通过靶向下调miR-423-5p抑制HSC活化,具有护肝作用。Luo,X等[30]用重组腺相关病毒8(rAAV8)抑制miR-96可减弱小鼠的肝纤维化并且防止血吸虫感染后的致死性,推断rAAV8介导的miR-96抑制可作为治疗肝血吸虫病的治疗策略。
miRNAs作为表观遗传调控机制的一种形式,已成为当前生命科学领域的前沿热点,有着重要的应用前景。miRNAs通过对HSC及相关信号通路或信号分子的影响,在HSC的活化、增殖和凋亡中充当调控者的角色[2-27]。了解miRNAs的调控机制,可为HF临床诊断及药物疗效评估提供新的生物标志物,为HF诊断及治疗提供新的研究思路。然而,肝纤维化调节系统非常复杂,涉及多种细胞和细胞因子的协同作用。许多miRNAs的作用靶点及调节通路等尚未明确,具有特异性HF诊断或治疗意义的miRNAs亦很模糊,有待进一步深入研究。
参考文献
[1]Wu P,Zuo X,Ji A.Stroke-induced microRNAs:The potential therapeutic role for stroke[J].Exp Ther Med,2012,3(4):571-576.
[2]Zhu Jie,Zhang Ziqiang,Zhang Yitong,et al.MicroRNA-212 activates hepatic stellate cells and promotes liver fibrosis via targeting SMAD7[J].Biochemical and biophysical research communications,2018,496(1):176-183.
[3]Wang,Y;Du,J;Niu,X;et al.MiR-130a-3p attenuates activation and induces apoptosis of hepatic stellate cells in nonalcoholic fibrosing steatohepatitis by directly targeting TGFBR1 and TGFBR2[J].Cell Death Dis,2017,8(5):e2792.
[4]Yu,F;Guo,Y;Chen,B;et al.MicroRNA-17-5p activates hepatic stellate cells through targeting of Smad7[J].Lab Invest,2015,95(7):781-9.
[5]Cheng,B;Zhu,Q;Lin,W;et al.MicroRNA-122 inhibits epithelial-mesenchymal transition of hepatic stellate cells induced by the TGF-β1/Smad signaling pathway[J].Exp Ther Med,2019,17(1):284-290.
[6]Wei,S;Wang,Q;Zhou,H;et al.miR-455-3p Alleviates Hepatic Stellate Cell Activation and Liver Fibrosis by Suppressing HSF1 Expression[J].Mol Ther Nucleic Acids,2019,16:758-769.
[7]Wang,P;Lei,S;Wang,X;et al.MicroRNA-134 Deactivates Hepatic Stellate Cells by Targeting TGF-βActivated Kinase 1-Binding Protein 1[J].Biochem Cell Biol,2019.
[8]Ma L,Yang X,Wei R,et al.MicroRNA-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression[J].Cell Death&Disease,2018,9(7):718.
[9]Sun J,Zhang H,Li L,et al.MicroRNA-9 limits hepatic fibrosis by suppressing the activation and proliferation of hepatic stellate cells by directly targeting MRP1/ABCC1[J].Oncology Reports,2017,37(3):1698-1706.
[10]Yu,F;Lu,Z;Huang,K;et al.MicroRNA-17-5p-activated Wnt/β-catenin pathway contributes to the progression of liver fibrosis[J].Oncotarget,2016,7(1):81-93.
[11]Yu,F;Fan,X;Chen,B;et al.Activation of Hepatic Stellate Cells is Inhibited by microRNA-378a-3p via Wnt10a[J].Cell Physiol Biochem,2016,39(6):2409-2420.
[12]Zhou,DD;Wang,X;Wang,Y;et al.MicroRNA-145 inhibits hepatic stellate cell activation and proliferation by targeting ZEB2 through Wnt/β-cateninpathway[J].Mol Immunol,2016,75:151-60.
[13]Yang,J;Lu,Y;Yang,P;et al.MicroRNA-145 induces the senescence of activated hepatic stellate cells through the activation of p53 pathway by ZEB2[J].J Cell Physiol,2019,234(5):7587-7599.
[14]Zou,Y;Cai,Y;Lu,D;et al.MicroRNA-146a-5p attenuates liver fibrosis by suppressing profibrogenic effects of TGFβ1 and lipopolysaccharide[J].Cell Signal,2017,39:1-8.
[15]Hyun,J;Wang,S;Kim,J;et al.MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression[J].Nat Commun,2016,7:10993.
[16]Zhang,T;Hu,J;Wang,X;et al.MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-κB-TNFαpathway[J].J Hepatol,2019,70(1):87-96.
[17]Wang,J;Chu,ES;Chen,HY;et al.microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway[J].Oncotarget,2015,6(9):7325-38.
[18]Huang,Y;Fan,X;Tao,R;et al.Effect of miR-182 on hepatic fibrosis induced by Schistosomiasis japonica by targeting FOXO1 through PI3K/AKT signaling pathway[J].J Cell Physiol,2018,233(10):6693-6704.
[19]Lei,Y;Wang,QL;Shen,L;et al.MicroRNA-101 suppresses liver fibrosis by downregulating PI3K/Akt/mTOR signaling pathway[J].Clin Res Hepatol Gastroenterol,2019.
[20]Yang,L;Dong,C;Yang,J;et al.MicroRNA-26b-5p Inhibits Mouse Liver Fibrogenesis and Angiogenesis by Targeting PDGF Receptor-Beta[J].Mol Ther Nucleic Acids,2019,16:206-217.
[21]Lin Yen-Cheng,Wang Feng-Sheng,Yang Ya-Ling,et al.MicroRNA-29a mitigation of toll-like receptor 2 and 4 signaling and alleviation of obstructive jaundice-induced fibrosis in mice[J].Biochemical and biophysical research co mmunications,2018,496(3):880-886.
[22]Yang,YL;Kuo,HC;Wang,FS;et al.MicroRNA-29a Disrupts DNMT3b to Ameliorate Diet-Induced Non-Alcoholic Steatohepatitis in Mice[J].Int J Mol Sci,2019,20(6).
[23]Huang,YH;Yang,YL;Huang,FC;et al.MicroRNA-29a mitigation of endoplasmic reticulum and autophagy aberrance counteracts in obstructive jaundice-induced fibrosis in mice[J].Exp Biol Med(Maywood),2018,243(1):13-21.
[24]Jing,F;Geng,Y;Xu,XY;et al.MicroRNA29a Reverts the Activated Hepatic Stellate Cells in the Regression of Hepatic Fibrosis through Regulation of ATPase H?Transporting V1 Subunit C1[J].Int J Mol Sci,2019,20(4).
[25]Genz,B;Coleman,MA;Irvine,KM;et al.Overexpression of miRNA-25-3p inhibits Notch1 signaling and TGF-β-induced collagen expression in hepatic stellate cells[J].Sci Rep,2019,9(1):8541.
[26]He,Y;Hwang,S;Cai,Y;et al.MicroRNA-223 Ameliorates Nonalcoholic Steatohepatitis and Cancer by Targeting Multiple Inflammatory and Oncogenic Genes in Hepatocytes[J].Hepatology,2019.
[27]Ma,L;Ma,J;Ou,HL;MicroRNA-219 overexpression serves a protective role during liver fibrosis by targeting tumor growth factorβreceptor 2[J].Mol Med Rep,2019,19(3):1543-1550.
[28]Nan,Y;Niu,X;Wang,R;et al.microRNA-1273g-3p is a useful non-invasive test for the prediction of liver fibrosis in patients with chronic hepatitis C[J].Exp Ther Med,2019,17(3):1817-1824.
[29]Feng,MH;Li,JW;Sun,HT;et al.Sulforaphane inhibits the activation of hepatic stellate cell by miRNA-423-5p targeting suppressor of fused[J].Hum Cell,2019.
[30]Luo,X;Zhang,D;Xie,J;et al.MicroRNA-96 Promotes Schistosomiasis Hepatic Fibrosis in Mice by Suppressing Smad7[J].Mol Ther Methods Clin Dev,2018,11:73-82.
关注SCI论文创作发表,寻求SCI论文修改润色、SCI论文代发表等服务支撑,请锁定SCI论文网! 文章出自SCI论文网转载请注明出处:https://www.lunwensci.com/yixuelunwen/14463.html