SCI论文(www.lunwensci.com):
摘要:在大鼠产后早期Tribbles假激酶-3(Trib3)高表达,Trib3在调节物质代谢、正常的生理过程和疾病的发生中起着非常重要的作用,其作用往往通过信号通路来完成。Trib3通过PI3K-Akt信号通路参与多种物质代谢过程,其中PI3K-Akt–mTORC1-p70S6K/4EBP1参与调节睾丸增殖、分化、精子发生,Trib3是Akt的抑制剂,因此以Akt为切入点,推断出Trib3-Akt-mTORC1-p70S6K/4EBP1信号通路可能调控精原干细胞增殖、分化及精子发生的过程,这个观点可为男性不育分子机制的研究提供新思路。
关键词:Tribbles假激酶-3;信号通路;睾丸增殖;精子发生
本文引用格式:秦承霞,张步洲,张楠,等.TRIB3-AKT-mTORC1-p70S6K/4EBP1信号通路可能参与调节大鼠精原干细胞和精子发生过程的研究进展[J].世界最新医学信息文摘,2019,19(100):57-60.
TRIB3-AKT-mTORC1-p70S6K/4EBP1 Signaling Pathway May be Involved in the Regulation of Rat Spermatogonial Stem Cells and Spermatogenesis
QIN Cheng-xia 1,2,ZHANG Bu-zhou 1,ZHANG Nan 1,YAN Huan-yu1,GAO Li-fei1,GAO Jie1,ZHANG Hui-min1,LIU Tao-di 1*(1.Department of Psychosomatic Medicine,Inner Mongolia Medical University,Hohhot Inner Mongolia;2.Department of Internal Medicine,Inner Mongolia Autonomous Region Maternal and Child Health Hospital,Hohhot Inner Mongolia)
ABSTRACT:Tribbles pseudo-kinase-3(Trib3)is highly expressed in the early postpartum period of rats.Trib3 plays an important role in regulating substance metabolism,normal physiological processes and disease occurrence,and its effect is often accomplished through signaling pathways.Trib3 is involved in the metabolism of various substances
through the PI3K-Akt signaling pathway.PI3K-Akt-mTORC1-p70S6K/4EBP1 is involved in the regulation of testicular proliferation,differentiation,and spermatogenesis.Trib3 is an inhibitor of Akt.Therefore,using Akt as the entry point,it is concluded that the Trib3-Akt-mTORC1-p70S6K/4EBP1 signaling pathway may regulate the process of sperm cell proliferation,differentiation and spermatogenesis.This view may provide new ideas for the study of the molecular mechanism of male infertility.
KEY WORDS:Tribbles pseudokinase-3;Signaling pathway;Testicular proliferation;Spermatogenesis
0引言
男性不育症发生率为10%左右。单纯男方因素约为30%,男性不育可能是由于身体发育异常、免疫原因、精子畸形、精子染色体异常、精子的基因缺失等多种原因造成的[1]。研究影响精子形成的因素,对男性不育有重要意义。Trib3是果蝇成虫的哺乳动物同源物,在大鼠精子发生过程中表达较高[2],同时被认为是Akt活性的抑制剂[3],而PI3K-Akt-mTOR信号通路可调控细胞的生长、增殖、分化和存活等生命过程[4];本文针对Trib3可能通过TRIB3-AKT-MTORC1-p70S6K/4EBP1信号通路参与调节精子的发生来进行综述。
1Trib3的特征
Tribbles假激酶-3(TRIB3;也称为TRB3,NIPK和SKIP3),属于假性激酶的tribbles家族,最初在果蝇中被描述为早期胚胎发生中细胞分裂的负调节因子[5]。Trib3是假蛋白酶,类似蛋白激酶,具有催化活性的支架。研究表明它是通过结合和调节几种关键蛋白质的活性实现其功能,包括蛋白激酶Akt和转录因子ATF4、CHOP和NF-κB,同时Trib3通过参与信号通路的组成而发挥其调节作用[6]。Trib3诱导条件包括正常的生理学、病理学条件或药理学作用。已知诱导Trib3的应激包括ER应激(ER腔中未折叠蛋白的积累)[7,8,9],氧化应激[7,10,11],缺氧[12,13],必需氨基酸缺乏[14],葡萄糖缺乏[15],葡萄糖过量[16]和游离脂肪酸过量[17]。总之,有大量文献表明Trib3 mRNA表达水平在广泛的、不同的应激条件下受到上调[6]。刘陶迪等通过基因芯片检测,筛查出Trib3在精子发生过程中差异性表达高达20.3倍[2]。本文想探讨Trib3在调节精子生成过程中的信号通路。
2Akt的结构与功能
Akt(PKB),为AGC蛋白激酶家族成员,与PKA及PKC具有高度的同源性。在同源蛋白PKA、PKC及逆转录病毒v-Akt(viral-Akt)的基础上,人类首次发现Akt蛋白[18]。目前已知的Akt家族成员有Akt1、Akt2、Akt3的3种亚型,研究发现,Akt的不同亚型在结构和功能方面均具有高度一致性,只是在不同肿瘤中的表达水平有所不同[19,20]。所有的Akt亚型都由1个氨基末端的PH结构域、中部的激酶结构域(ATP结合域)以及羧基末端的调节结构域组成,并且3种亚型的活化方式也高度一致。Akt的PH结构域能够与上游PI3K产生的第二信使4,5-二磷酸磷脂酰肌醇及其磷酸化后产生的PIP3以相对较高的亲和力特异性地结合,介导Akt定位于膜上并使其磷酸化[21],其对Akt的活化起重要作用。Akt是PI3K的下游效应子,被PI3K激活后进一步激活其下游因子,如FOXO1、mTOR、NF-kB、GSK-3β等,对细胞增殖、凋亡产生调控作用[22]。
3TRIB3通过PI3K-Akt途径参与多种生理代谢过程
3.1TRIB3通过PI3K-Akt途径调节葡萄糖代谢。Trb3是果蝇tribbles的同源基因,在肝脏、脂肪组织、心脏、肾脏、肺、皮肤、小肠、胃和其他组织中表达。在禁食和糖尿病的情况下被认为是Akt活性的抑制因子。PI3K/Akt途径主要介导胰岛素对蛋白质、碳水化合物和脂质的合成和储存的合成代谢作用[3]。该途径主要在哺乳动物中起作用,是一种进化上保守的信号盒,根据生长因子的刺激和营养物质的可利用性来传递生存信号[3]。肝Akt是调节肝葡萄糖输出的胰岛素信号的关键介质,因此,调节Akt活性的蛋白质对葡萄糖稳态有显著影响。随后的研究表明TRB3是Akt活性的重要调节器,TRB3主要在肝脏通过直接结合Akt并阻断其激活,抑制胰岛素信号传导[23]。因此,TRIB3通过PI3K-Akt途径调节葡萄糖代谢。
3.2TRIB3依靠PI3K/Akt信号通路调节在成骨分化过程中的表达。人骨髓间充质干细胞(hBMSCs)中的成骨分化受各种因素的调节,包括骨形态发生蛋白(BMP),Notch,生长激素和丝裂原活化蛋白激酶(MAPK)。Tribbles同源物3(TRIB3)是一种假激酶,张翠等发现TRIB3在hBMSCs的成骨分化过程中高度表达,并且TRIB3在调节hBMSC中的增殖和成骨分化中起重要作用[24]。局部粘附激酶(FAK)或磷脂酰肌醇3激酶(PI3K)的抑制导致Trib3表达显著下降,通过激活(PI3K/AKT)信号,增加胰岛素样生长因子-1(IGF-1)来恢复Trib3的表达[24]。在hBMSC成骨分化过程中PI3K/Akt信号通路的激活介导了Trib3的蛋白表达。Trib3依靠PI3K/Akt信号通路调节在成骨分化过程中的表达。
3.3TRIB3介导的PI3K/AKT/mTOR靶向和自噬级联调节细胞内应激。肥胖,动脉粥样硬化和糖尿病等代谢性疾病通常与侵袭性癌症的风险增加有关。有证据表明,在代谢疾病和癌症中,未折叠蛋白反应(UPR)和内质网应激(ERS)途径均被调节。UPR还引发与PI3K/AKT/mTOR和自噬途径的协调信号传导,以促进应激适应机制[25]。然而,不受控制的UPR和由此产生的ERS使细胞升高至代谢功能障碍和最终细胞死亡。TRIB3的“假激酶”功能促进多种转录因子和信号蛋白的失活[25]。TRIB3的MEK1结合域使其能够使多种MAP激酶失活;TRIB3的COP1基序有助于大量TRIB3相关蛋白的泛素化和蛋白酶体降解。TRIB3最良好的作用是PI3K/AKT/mTOR途径,其中TRIB3介导的AKT磷酸化抑制降低了胰岛素信号传导和细胞存活。相反,癌细胞可以通过抑制TRIB3表达来上调AKT存活途径或改变TRIB3定位以降解诱导核转录因子如C/EBPa和PPARg的分化[25]。TRIB3作为关键的“应激调节开关”,将体内平衡、代谢疾病和癌症联系起来。在胞质和线粒体蛋白,TRIB3介导的PI3K/AKT/mTOR靶向和自噬级联作为关键的因素调节细胞内应激[25]。PI3K/AKT/mTOR通路在调节正常代谢功能中至关重要[26]。
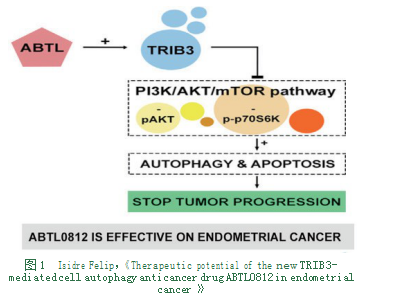
3.4Trib3通过PI3K/AKT/mTOR途径在子宫内膜癌发挥作用。TRIB3是一种高度保守的蛋白质,属于Tribbles假基因家族,参与多种生理过程,比如葡萄糖调节,肿瘤细胞迁移,自噬和细胞周期控制[27]。PI3K/AKT/mTOR通路在子宫内膜癌(EC)中经常过度激活。ABTL0812是一种小分子抑制剂,具有抑制该通路的独特机制,是高风险子宫内膜癌患者的有效治疗选择[27]。ABTL0812诱导TRIB3表达上调,导致EC3细胞上的PI3K/AKT/mTOR轴抑制和自噬细胞死亡(如图1)[27]。TRIB3 mRNA可用作药效学生物标志物来监测ABTL0812的疗效。因此,抗肿瘤药ABTL0812可以通过在EC患者中诱导TRIB3表达抑制PI3K/AKT/mTOR轴发挥治疗作用。
3.5PI3K-AKT-MTORC1-p70S6K/4EBP1在雄性生殖中的作用。mTOR蛋白属于磷脂酰肌醇激酶相关蛋白激酶家族,进化上相对保守,是细胞营养、能量状态的传感器[28]。在代谢和环境压力等作用下,mTOR信号可激活蛋白质翻译、参与调节蛋白质合成和能量代谢,是细胞增殖、分化和生长过程的中心调控者,但mTOR功能正常有效地得以发挥需以合成mTOR复合物1(mTORCl)和mTOR复合物2(mTORC2)为前提[29]。mTORC1和mTORC2的结构均类似于二聚体(如图2),分别以正负调控因子形式发挥其功能[30]。研究发现PI3K-mTOR信号分子可调控睾丸组织中p70S6K和4EBP1表达来调节精原干细胞的增殖和精子发生[31]。在精原干细胞分化过程中,激活PI3K-AKT-mTOR信号通路可增加Kit蛋白合成,且PI3K-Akt-mTOR信号通路与Kit可能存在反馈调节以调控精子发生[32]。运用基因敲除技术敲除LKB1、TSC1和TSC2后可导致小鼠睾丸精子细胞减少、破环精原干细胞极性和睾丸细胞紧密连接功能和组织完整性,同时LKB1基因敲除抑制小鼠睾丸组织中AMPK信号表达[33]。以上研究提示,PI3K-Akt-mTOR和LKB1-AMPK-mTOR通路很可能参与调控睾丸发育和精子发生[34],但具体机制和功能仍有待深入研究。
4提出假说:TRIB3-AKT-mTORC1-p70S6K/4EBP1信号通路
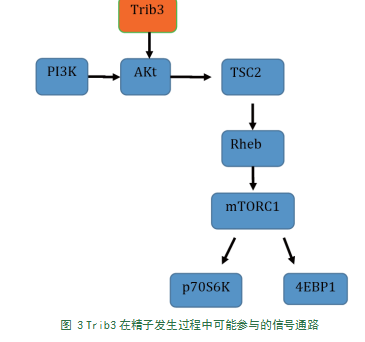
Trib3是Akt活性的抑制剂[3],而Akt位于PI3K-Akt-mTOR信号通路的核心位置,在调控细胞凋亡、增殖和代谢等多种生理活动中发挥重要功能。在精子发生过程中,mTOR信号通路调控精原干细胞的增殖分化与自我更新,且可能参与调节精母细胞的减数分裂[35]。精原干细胞和精母细胞均有mTOR、p70S6K、4EBP1及相应磷酸化蛋白(p-mTOR、p-p70S6K、p-4EBP1)的表达,且mTOR信号对早期生精细胞和精原干细胞表型维持及分化有重要作用[36]。精子发生过程包括精原干细胞的增殖分化、精母细胞减数分裂和精子形成[37]。体内研究证实mTOR信号调控精原干细胞减数分裂起始和增殖、精原细胞的增殖和分化[36]。通过抑制mTORC1通路可阻断精原细胞分化并诱导未分化精原细胞在生精小管内累积,且睾丸组织中细胞分化调节因子酪氨酸激酶受体c-Kit(Kit)等编码基因翻译受阻[38]。在小鼠精原细胞向精母细胞分化阶段,细胞内mRNA翻译及其调控过程依赖于mTOR信号[39]。因此推断mTOR信号分子在睾丸细胞增殖分化和生精过程中有着不可或缺的作用[34]。根据文献资料,我们提出假说:Trib3可能通过Trib3-Akt-TSC2-Rheb-mTORC1-p70S6K/4EBP1信号通路调控精原干细胞增殖、分化及精子发生过程(如图3)。
5小结与展望
根据以往的文献报道得知,Trib3通过PI3K-Akt轴参与多种生理过程。PI3K-Akt–TSC2-Rheb-mTORC1-p70S6K/4EBP1在精子发生过程中发挥重要作用,因此推断出Trib3可能以Akt为切入点,通过Trib3-Akt-mTORC1-p70S6K/4EBP1信号通路调控精原干细胞增殖、分化及精子发生过程。Trib3在大鼠精原干细胞及精子发生过程中的研究还未见报道,有待于进一步研究证实。有理由相信,随着实验的深入和对相关机制的阐释,Trib3-Akt–mTORC1-p70S6K/4EBP1信号通路有希望成为治疗男性生殖功能低下和男性不育的重要靶点。
参考文献
[1]计垣.从不同角度探讨男性不育的原因[J].中国优生与遗传杂志,2013,21(10):136-139.
[2]Yan Zhang,Fenhua Luo,Sachula Wu,et al.Tribbles homolog 3 expression in spermatogonial stem cells of rat testes[J].Cell,2014,38:1403–1407.
[3]Haruka Okamoto,Esther Latres,Rong Liu,et al.Genetic Deletion of Trb3,the Mammalian Drosophila tribbles Homolog,Displays Normal Hepatic Insulin Signaling and Glucose Homeostasis[J].Diabetes,2007,56(5):1350-6.
[4]Dibble CC,Cantley LC.Regulation of mTORC1 by PI3K signaling[J].Trends Cell Biol,2015,25(9):545-555.
[5]Zeqiraj E,van Aalten DM.Pseudokinases-remnants of evolutionor key allosteric regulators?[J].Curr Opin Struct Biol,2010,20:772–81.
[6]TiitÖrd,TõnisÖrd.Mammalian Pseudokinase TRIB3 in Normal Physiology and Disease:Charting the Progress in Old and New Avenues[J].Current Protein and Peptide Science,2017,18:819-842.
[7]Örd,D.,Örd,T.Mouse NIPK interacts with ATF4 and affects its transcriptional activity[J].Exp.Cell Res,2003,286:308-320.
[8]Örd,D.,Örd,T.Characterization of human NIPK(TRB3,SKIP3)gene activation in stressful conditions.Biochem.Biophys[J].Res.Commun,2005,330:210-218.
[9]Ohoka,N.,Yoshii,S.,Hattori,T.,et al.TRB3,a novel ER stress-inducible gene,is induced via ATF4-CHOP pathway and is involved in cell death[J].EMBO J,2005,24:1243-1255.
[10]Morse,E.,Schroth,J.,You,Y.H.,et al.TRB3 is stimulated in diabetic kidneys,regulated by the ER stress marker CHOP,and is a suppressor of podocyte MCP-1.Am.J.Physiol.Renal[J].Physiol,2010,299:965-972.
[11]Lange,P.S.,Chavez,J.C.,Pinto,J.T.,et al.ATF4 is an oxidative stress-inducible,prodeath transcription factor in neurons in vitro and in vivo[J].J.Exp.Med,2008,205:1.
[12]Bowers,A.J.,Scully,S.,Boylan,J.F.SKIP3,a novel Drosophila tribbles ortholog,is overexpressed in human tumors and is regulated by hypoxia[J].Oncogene,2003,22:2823-2835.
[13]Wennemers,M.,Bussink,J.,Scheijen,B.,et al.Tribbles homolog 3 denotes a poor prognosis in breast cancer and is involved in hypoxia response[J].Breast Cancer Res,2011,13:R82.
[14]Örd,T.,Innos,J.,Lilleväli,K.,et al.Trib3 is developmentally and nutritionally regulated in the brain but is dispensable for spatial memory,fear conditioning and sensing of amino acid-imbalanced diet[J].PLoS One,2014,9:e94691.
[15]Schwarzer,R.,Dames,S.,Tondera,D.,et al.TRB3 is a PI 3-kinase dependent indicator for nutrient starvation[J].Cell Signal,200618:899-909.
[16]Liu,J.,Wu,X.,Franklin,J.L.,et al.Mammalian Tribbles homolog 3 impairs insulin action in skeletal muscle:role in glucose-induced insulin resistance[J].Am.J.Physiol.Endocrinol.Metab,2010,298:565-576.
[17]Morse,E.,Schroth,J.,You,Y.H.,et al.TRB3 is stimulated in diabetic kidneys,regulated by the ER stress marker CHOP,and is a suppressor of podocyte MCP-1[J].Am.J.Physiol.Renal.Physiol.,2010,299:965-972.
[18]ZHAO L R,DU Y J,CHEN L L,et al.Mentin-1 promotes the growth of neural stem cells via activation of Akt signaling[J].Mol Med Rep,2015,11(3):1859-1864.
[19]THOMAS C C,DEAK M,ALESSI D R.High-resolution structure of the pleckstrin homology domain of protein kinase B/Akt bound to phosphatidylinositol(3,4,5)-trisphosphate[J].Curr Biol,2002,12(14):1256-1262.
[20]MAFFUCCI T,FAL ASCA M.Specificity in pleckstrin homology(PH)domain membrane targeting:a role for a phosphoinositide-protein co-operative mechanism[J].FEBS Lett,2001,506(3):173-179.
[21]BERGES C,BEDKE T,STUEHLER C,et al.Combined PI3K/Akt and Hsp90 targeting synergistically suppresses essential functions of alloreactive T cells and increases Tregs[J].J Leukoc Biol,2015,98(6):1091-1105.
[22]刘静.Akt基因表达对卵巢上皮性癌细胞增殖的影响[D].河北医科大学,2018.
[23]Du K,Herzig S,Kulkarni RN,et al.TRB3:a tribbles homolog that inhibits Akt/PKB activation by insulin in liver[J].Science 300:1574–1577,2003.
[24]Cui Zhang,Fan-Fan Hong,Cui-Cui Wang,et al.TRIB3 inhibits proliferation and promotes osteogenesis in hBMSCs by regulating the ERK1/2 signaling pathway[J].Sci Rep,2017,7:10342.
[25]Debasis Mondal AditiMathur Partha K.Chandra.Tripping on TRIB3 at the junction of health,metabolic dysfunction and cancer.Biochimie[J].Volume,2016,124:34-52.
[26]Salazar M,Lorente M,García-Taboada E,et al.Loss of Tribbles pseudokinase-3 promotes Akt-driven tumorigenesis via FOXO inactivation[J].Cell Death Differ,2015,22(1):131-44.
[27]Isidre Felip,Cristian Pablo Moiola,CristinaMegino-Luque
,et al.Therapeutic potential of the new TRIB3-mediated cell autophagy anticancer drug ABTL0812 in endometrial cancer[J].Gynecologic Oncology,2019,153:425–435.
[28]Hwang SK,Kim HH.The functions of mTOR in ischemic diseases[J].BMB Rep,2011,44(8):506-511.
[29]Perl A.Activation of mTOR(mechanistic target of rapamycin)in rheumatic diseases[J].NatRevRheumatol,
2016,12(3):169-182.
[30]Perl A.mTOR activation is a biomarker and a central pathway to autoimmune disorders,cancer,obesity,and aging[J].Ann N Y Acad Sci,2015,1346(1):33-44.
[31]Xu H,Shen L,Chen X,et al.mTOR/P 70S 6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats[J].Reprod Biomed Online,2016,32(2):207-217.
[32]Busada JT,Chappell VA,Niedenberger BA,et al.Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse[J].Dev Biol,2015,397(1):140-149.
[33]Tanwar PS,Kaneko-Tarui T,Zhang L,et al.Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis[J].Hum Mol Genet,2012,21(20):4394-4405.
[34]段鹏,全超,黄文婷等.PI3K-Akt/LKB1-AMPK-mTOR-p70S6K/4EBP1信号通路参与调节睾丸发育和精子发生的研究进展[J].中华男科学杂志,2016,22(11):1016-1020.
[35] Xu H,Shen L,Chen X,et al.mTOR/P 70S6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats[J].Reprod Biomed Online,2016,32(2):207-217.
[36]Sahin P,Sahin Z,Gungor-Ordueri NE,et al.Inhibition of mammalian target of rapamycin signaling pathway decreases retinoic acid stimulated gene 8 expression in adult mouse testis[J].Fertil Steril,2014,102(5):1482-1490.
[37]徐晨,宋宁.精子发生过程中的表观遗传学调控[J].中华男科学杂志,2014,20(5):387-391.
[38]Busada JT,Niedenberger BA,Velte EK,et al.Mammalian target of rapamycin complex 1(mTORC1)is required for mouse spermatogonial differentiation in vivo[J].Dev Biol,2015,407(1):90-102.
[39]Mes sina V,D i SA,P edr otti S,e t al.Differ ential contribution of the MTOR and MNK pathways to the regulation of mRNA translation in meiotic and postmeiotic mouse male germ cells[J].BiolReprod,2010,83(4):607-615.
关注SCI论文创作发表,寻求SCI论文修改润色、SCI论文代发表等服务支撑,请锁定SCI论文网! 文章出自SCI论文网转载请注明出处:https://www.lunwensci.com/yixuelunwen/25410.html