摘要:肠道菌群已被发现可通过微生物-肠-脑轴与大脑之间的相互作用,对各种生理过程进行调节。同时,微生物-肠-脑轴在抑郁症、孤独症谱系障碍、双相情感障碍、雷特综合征和注意缺失障碍等精神疾病中具有显著作用。在此背景下,文章就微生物-肠-脑轴在精神疾病中的研究进展进行综述,并展望了该领域未来的发展方向。
关键词:抑郁症,孤独症谱系障碍,双相情感障碍,雷特综合征,注意缺陷障碍,微生物-肠-脑轴,肠道菌群
精神疾病是一类可引起认知、情感、精神、行为等方面异常的功能障碍性疾病,主要症状包括意识障碍、运动障碍、语言和言语障碍、认知功障碍及情感障碍[1]。美国精神医学学会在对现有精神疾病整理分类后发现,在400种疾病中,全球患病人数较多的有抑郁症、孤独症谱系障碍、双相情感障碍、雷特综合征和注意缺陷障碍等。
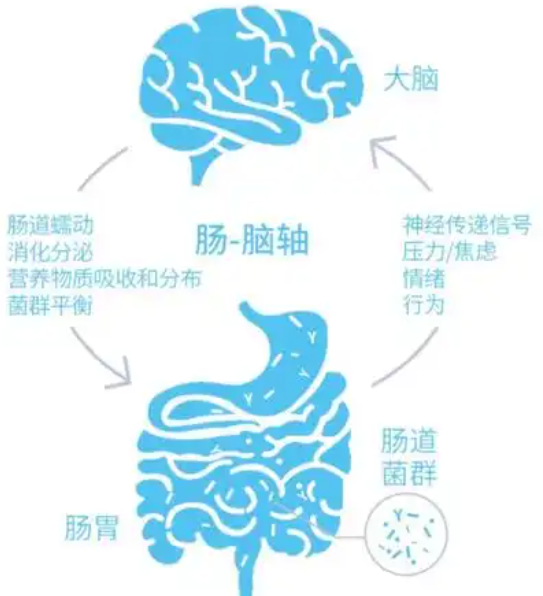
大量研究发现,肠道与大脑以双向的方式相互作用,被称为肠-脑轴。在肠道内,作为肠-脑轴的关键调节器—常驻微生物群落,引起了研究者的广泛关注。人体内包含至少160种优势菌群,且大多数菌群属于厚壁菌门和拟杆菌门,占肠道菌群总数的90%以上;其次是放线菌门、梭杆菌门、变形菌门和疣微菌门等[2]。肠道菌群主要参与机体的生长发育和衰老过程,具有改善人体新陈代谢、调节免疫机制、抗炎、抗氧化和抗衰老等功能。肠道菌群可通过多种途径参与胃肠道和中枢神经系统(CNS)间的双向交流;反之,大脑也可调节肠道菌群的结构组成,并维持胃肠道环境的平衡。由此,产生了一个新的生物概念,即微生物-肠-脑轴。
微生物-肠-脑轴间的相互作用主要通过3种途径进行,分别为免疫通路、神经元通路和内分泌系统通路,且这3种途径间存在相互作用和交叉关系[3]。肠道微生物群的数量和肠道菌群失调会引发人体诸多异常情况,如消化和吸收紊乱、维生素生产或代谢紊乱、消化脂肪困难、肠道屏障破坏,以及对免疫系统的过度刺激等[4-5]。现有研究虽已探讨了肠道微生物群影响的生理和病理机制,但受限于肠道菌群的复杂性,目前对于复杂疾病影响的研究尚未完善,特别是在对精神疾病的作用方面。近年来,微生物-肠-脑轴在精神疾病相关领域中的研究不断增多,为复杂神经精神疾病的治疗提供了新思路和方法。基于此,本文将对微生物-肠-脑轴在精神疾病中的研究进展进行综述。
1微生物-肠-脑轴与抑郁症
抑郁症作为一种常见的精神障碍状态,主要表现为悲伤和冷漠。抑郁症涉及社会、心理和生理因素的综合作用,如生活的重大变化、家庭问题、慢性健康问题或成瘾等[6]。世界卫生组织相关数据显示,全世界每年约有2.8亿人患有重度抑郁症。
微生物-肠-脑轴可参与抑郁症的发病机制,其肠道代谢物、微生物细胞成分甚至肠道菌群都与抑郁症的发病存在关联。研究表明,抑郁症患者体内存在肠道菌群紊乱,与正常人群相比,微生态严重失衡。具体表现为厚壁菌门、放线菌门和拟杆菌门的丰度增加,同时拟杆菌属/厚壁菌属比率提高,即拟杆菌属增加,而细菌属、粪杆菌属和粪球菌属则减少[7-11]。此外,短链脂肪酸、丁酸钠等微生物代谢产物也会对抑郁症产生影响。丁酸钠具有抗抑郁作用,而抑郁症患者的肠道中产生丁酸盐代谢物的菌群相对较少,使得丙酸和异丙酸等短链脂肪酸的含量显著低于正常对照人群[12]。微生物细胞成分如脂多糖等,可经由革兰氏阴性细菌和周边炎症信号到达大脑,穿越血脑屏障引起神经炎症,随后通过慢性激活特定细胞,包括突触缺陷、脱髓鞘、异常神经发生和神经递质释放,参与抑郁症的发病机制[13-14]。微生物信号、病理性神经生物学变化和抑郁情绪可激活下丘脑-垂体-肾上腺轴,促进皮质醇的合成与释放。过量的皮质醇通过调节肠道屏障功能和炎症反应,可促进肠道病理,导致肠道渗漏[15],而这一过程正是抑郁症患者微生物-肠-脑轴的关键环节。同时,肠道代谢物、微生物细胞成分,甚至微生物群也可通过受损的肠道屏障加剧炎症反应。例如,Th17/调节性T细胞(Th17/Treg)失衡、白细胞介素IL-6、IL-1β和肿瘤坏死因子TNF-α等成分均与抑郁症的发病机制有关[16]。LI等[17]从抑郁症患者的粪便中分离出了可表达3β-羟基类固醇脱氢酶降解睾酮的分枝杆菌,并发现给大鼠注射该菌株会诱发抑郁行为。
与宿主的基因组不同,肠道微生物群是动态、多样化的,且能够从外部进行调节。饮食控制、补充活的微生物(单一或混合的物种),以及服用有利于微生物群生长的分子物质,都可控制微生物的组成和功能,保持肠道内菌群的健康平衡。这突出了肠道微生物群作为抑郁症一个新的治疗靶点的可能性。目前,已有多项研究强调了各种微生物靶点疗法在缓解抑郁症方面的疗效,包括饮食干预[18-21]、肠道菌群移植[22-27]、益生菌和益生元[8,28-34]等。
2微生物-肠-脑轴与孤独症谱系障碍
孤独症谱系障碍(ASD)是一种复杂的神经发育疾病,其特征是患者的社会互动和沟通受损,且存在重复和刻板的行为[35]。在过去几十年间,ASD的全球流行率急剧上升。迄今为止,该疾病在美国的发病率已达到1/36,且男孩的比例是女孩的3~5倍[36]。ASD的病因较为复杂,现有研究认为其发病和进展会受到遗传和环境因素的共同作用[37]。有高达48.67%的患者出现了腹痛、腹泻、便秘和胃食管反流等胃肠道并发症(GI),揭示了胃肠道及其寄生微生物在ASD发病和发展中的重要作用[38]。
当前,连接ASD和GI的多方面潜在机制虽尚未明确,但已有一些研究将肠道微生物群视作ASD和GI症状之间的潜在联系。事实上,在ASD患者中也确实发现了部分微生物群扰动。据报道,与正常对照人群相比,ASD患者粪便样本中梭菌属[39-40]、乳酸杆菌[41]、瘤胃球菌[42]和拟杆菌[43]的丰度显著增加;普雷沃氏菌属[44]、粪球菌属[44]和韦荣球菌科[44]、双歧杆菌属[45-46]物种的丰度减少。另有研究报道了梭菌属、肠球菌在ASD患儿粪便中的检出率明显高于对照组,主要包括葡萄球菌、念珠菌属和产气荚膜梭菌,且厚壁菌门/拟杆菌门比率有所提高[47]。在ASD儿童的粪便中发现,TNF-α水平升高,且其与GI症状的严重程度密切相关[48]。同时,TNF-α和IL-6的升高也可视作大脑炎症的标志物[49]。这些数据再次证实了ASD儿童大脑中炎症水平较高。综上,肠道微生物群可能是ASD发病机制中的促成因素。
肠道微生物群产物包括多种代谢产物,如短链脂肪酸(SCFAs)、酚类化合物和游离氨基酸等,且这些代谢产物会影响ASD患者的行为[50]。据报道,ASD患者的粪便样本中的SCFAs浓度较高[51]。短链脂肪酸包括乙酸、丁酸盐、异丁酸、戊酸和异戊酸,是肠道细菌发酵不可消化碳水化合物的产物;迷走神经是大脑与肠道通信的直接途径[52];其他微生物产物包括作为神经递质的化合物,可能影响突触功能和大脑的通信[53-54]。例如,5-羟色胺和其他微生物代谢物,如-氨基丁酸(GABA)等,可作为神经递质。现有研究证实,胃肠道疾病和肠道微生物群的变化会影响肠道和大脑中的血清素信号传导[55]。这表明,肠道微生物群与肠-脑轴间的相互作用对ASD有着重要影响。
基于肠道微生物群可操作的特性,重新平衡肠道微生物群被认为是治疗ASD的一种有希望的方法。现有研究表明,补充益生元和益生菌能够影响ASD患者的行为。具体而言,用双歧杆菌、瑞士乳杆菌、罗伊氏乳杆菌和副干酪乳杆菌治疗ASD儿童,可缓解共病GI问题[56];罗伊氏乳杆菌本身可逆转脂多糖(LPS)引起的肠道炎症[57];细菌罗伊氏乳杆菌能促进脑垂体后叶产生更多的催产素,而催产素是一种下丘脑激素,对ASD患者的社会行为具有积极影响[58];使用益生菌治疗ASD相关的行为特征也在ASD小鼠模型中得到了支持[59]。由此可见,以微生物为靶点并将其作用于神经、免疫和内分泌等通路,为ASD治疗提供了新的思路和方向。
3微生物-肠-脑轴与双向情感障碍
双相情感障碍(BD)是一种慢性和复发性神经疾病,其特征是反复出现躁狂或轻度躁狂发作,交替出现抑郁发作,同时也会加剧多系统并发症风险,包括认知障碍和代谢障碍,进而对患者的生活质量产生严重影响[60]。虽然BD的病理生理学仍需要更深入的阐明,但免疫炎症活性、氧化与亚硝化应激和神经调节色氨酸分解代谢产物的变化已被认为是BD的病因和病程[61]。BD患者肠道微生物组成的改变,证明肠道微生物生态失调有利于BD患者的疾病进展和病理生理学研究[62]。
研究发现,BD患者肠道内粪杆菌、拟杆菌、普氏菌、肠杆菌和梭状芽孢杆菌群丰度显著增加,而双歧杆菌与肠杆菌科的比例则有所降低[63]。另有研究表明,BD患者厚壁菌门和拟杆菌门的生物数量增加,而细菌门的生物数量减少,使得厚壁菌门与细菌门的比例相应提升[64-65]。虽然现有研究结果并不完全符合,但一致发现BD患者体内产丁酸的细菌数量减少,这是因为已知的通过产生SCFAs作用于认知功能的肠道微生物群,会影响中枢神经系统中的丁酸水平[66]。鉴于丁酸盐具有神经保护和认知功能改善作用,丁酸盐产生菌浓度不足,可能是因为其参与了BD患者认知功能障碍的病理生理过程。丁酸产生细菌与认知障碍之间的相互作用,表明微生物产生的小分子代谢物介导了宿主-微生物组的作用机制。对BD患者大脑转录组数据的分析,也显示出了肠道微生物代谢物的紊乱,包括色氨酸和SCFAs等。这些重叠的代谢物谱,揭示了肠道微生物群参与BD的潜在途径。
基于微生物-肠-脑轴参与BD的病理生理过程,益生元和益生菌等微生物群可改善BD的症状。临床研究表明,益生菌具有促进BD患者肠道微生物多样性和改善认知功能的作用。例如,乳酸菌株Bb12可降低BD患者再次住院的风险[67]。研究发现,长期使用益生菌补充剂(含有乳酸杆菌属和双歧杆菌属的生物)后,BD患者的注意力和精神运动处理速度均得到了显著提高[68]。
4微生物-肠-脑轴与雷特综合征
雷特综合征(RTT)是一种早发性神经发育障碍,主要由X染色体上的MeCP2基因突变引起。RTT主要影响女性,易造成严重的认知障碍和身体残疾,是女性智力障碍的最普遍原因之一。
胃肠道功能障碍和便秘在RTT患者中十分常见,意味着神经功能异常与肠道功能和肠道微生物群之间可能存在联系[69]。现有研究发现,RTT患者和MeCP2突变的动物模型的肠道微生物组的结构发生了改变,主要表现为丰度和多样性均有所减少[70-71]。RTT中放线菌门和厚壁菌门的相对丰度增加,而拟杆菌门的存在则减少,使得厚壁菌门/拟杆菌门的比例明显升高;拟杆菌、双歧杆菌、副拟杆菌、黑螺旋杆菌、黑胞杆菌、大肠杆菌、放线菌、肠球菌和梭菌在RTT中的相对丰度增加,而粪杆菌、链球菌、瘤胃球菌、普雷沃氏菌、吉米格菌和阿利斯蒂普菌的相对丰度减少[69-73],表明RTT与肠道生态失调有关。MeCP2的突变改变了肠道微生物群落结构,影响了RTT中SCFAs的产生和胃肠道病理生理学,易造成便秘、炎症和宿主细胞因子失调[70]。这种通过肠-脑轴介导的MeCP2损伤,表现为RTT患者的肠道生态失调。
在Me CP2基因缺陷小鼠中,小胶质细胞对谷氨酰胺摄取增加可由于海马神经元谷氨酸过量产生而引起毒性效应,导致树突和突触损伤[74-75]。谷氨酸是一种兴奋性神经递质,对肠道微生物高度敏感,可被细菌谷氨酸脱羧酶代谢产生Y-氨基丁酸(GABA)。另有研究表明,产生GABA的细菌可减少小鼠模型的抑郁和焦虑行为[76-77]。这些发现,为RTT治疗提供了前瞻性的思路,有可能改善患者的生理功能或通过肠道菌群调节影响疾病进展。
肠道微生物在RTT疾病进展中起着关键作用。RTT患者肠道菌群的变化,可能反映临床表现,并影响疾病演变。未来研究应关注恢复肠道菌群,或将特定的益生菌定植作为RTT治疗的一种新方法。
5微生物-肠-脑轴与注意缺陷多动障碍
注意缺陷多动障碍(ADHD)作为一种常见的神经发育障碍,临床表现为发育不当和损害、注意力不集中、多动和冲动。该种障碍常在儿童时期发病,且在男性群体中更为常见。据统计,该病症全球的患病率为5%[78]。多巴胺受体DRD4和DRD5基因,以及多巴胺和血清素递质基因,被认为是该疾病的主要病因[79]。多项证据表明,ADHD发病机制与微生物群-肠-脑轴有关[80-81]。研究发现,ADHD患者中放线菌门(如双歧杆菌等)丰度增加,而厚壁菌门丰度减少[82]。有趣的是,ADHD患者的微生物群表现出更显著的产生环己二烯基脱氢酶(CDT)的能力,而CDT可参与多巴胺前体(苯丙氨酸)的合成。
通过在生命早期补充益生菌,可降低患神经精神疾病的风险。PARTTY等[83]研究表明,早期给予鼠李糖乳杆菌可能会降低ADHD的风险。而乳杆菌则可通过迷走神经调节小鼠的情绪行为和中枢GABA受体的表达[84]。此外,摄入Ω-3不饱和脂肪酸也很重要,特别是二十二碳六烯酸(DHA)和二十碳五烯酸(EPA),对膜流动性、神经传递和受体功能有着至关重要的影响[85]。在一项基于ADHD动物模型的研究中发现,摄入富含Ω-3不饱和脂肪酸的饮食会降低小鼠的冲动性,同时提高小鼠的注意力[86]。
6总结与展望
肠道微生物及其代谢物在肠-脑轴的相互作用中发挥着至关重要的作用。特定的神经发育途径可能需要来自肠道微生物信号的反应,而肠道微生物群很可能对中枢神经系统的结构和功能产生持久影响[87]。精神疾病可破坏肠道微生物平衡;反之,肠道微生物失衡也会加快疾病进展。目前,肠道微生物与精神疾病的发病机制间的联系,成为科学领域的前沿研究。
调节肠道微生物可能会抑制精神疾病发展,增强宿主免疫力,从而增进人类和动物福祉。尽管如此,目前临床上关于饮食干预、肠道菌群移植、补充益生菌和益生元等治疗疾病的方法仍然有限,缺乏标准化的方案。因此,深入研究支持精神疾病和肠道微生物群的调控机制,不仅有利于肠道健康,而且对于扩大相关精神疾病的预防范围也具有深远意义。
参考文献
[1]WANG Q,YANG Q,LIU X.The microbiota gut brain axis and neurodevelopmental disorders[J].Protein&Cell,2023,14(10):762-775.
[2]GOLLWITZER E S,MARSLAND B J.Microbiota abnor-malities in inflammatory airway diseases-potential for therapy[J].Pharmacology&Therapeutics,2014,141(1):32-39.
[3]AGIRMAN G,HSIAO E Y.SnapShot:The microbio-ta-gut-brain axis[J].Cell,2021,184(9):2524-2524.
[4]SINGH R,ZOGG H,WEI L,et al.Gut microbial dysbio-sis in the pathogenesis of fastrointestinal dysmotility and metabolic disorders[J].Journal of Neurogastroenterology and Motility,2021,27(1):19-34.
[5]IEBBA V,TOTINO V,GAGLIARDI A,et al.Eubiosis and dysbiosis:The two sides of the microbiota[J].New Microbiology,2016,39(1):1-12.
[6]ZHOU L,FOSTER J A.Psychobiotics and the gut-brain axis in the pursuit of happiness[J].Neuropsychiatric Dis-ease and Treatment,2015,11:715-723.
[7]NASERIBAFROUEI A,HESTAD K,AVERSHINA E,et al.Correlation between the human fecal microbiota and depression[J].Journal of Neurogastroenterology and Motil-ity,2014,26(8):1155-1162.
[8]LIU L,WANG H,ZHANG H,et al.Toward a deeper un-derstanding of gut microbiome in depression:The promise of clinical applicability[J].Advanced Science(Weinheim,Baden-Wurttemberg,Germany),2022,9(35):e2203707.
[9]NIKOLOVA V L,SMITH M R B,HALL L J,et al.Per-turbations in gut microbiota composition in psychiatric disorders:A review and meta-analysis[J].JAMA Psychi-atry,2021,78(12):1343-1354.
[10]SIMPSON C A,DIAZ-ARTECHE C,ELIBY D,et al.The gut microbiota in anxiety and depression-A sys-tematic review[J].Clinical Psychology Review,2021,83:101943.
[11]YANG J,ZHENG P,LI Y F,et al.Landscapes of bacte-rial and metabolic signatures and their interaction in ma-jor depressive disorders[J].Science Advances,2020,6(49).
[12]KAROLINA S,ELZ.BIETA G,DOMINIKA M,et al.Fae-cal short chain fatty acids profile is changed in polish depressive women[J].Nutrients,2018,10(12):1939.
[13]JIANG Y S,LIU Y,GAO M Q,et al.Nicotinamide ribo-side alleviates alcohol-induced depression-like be-haviours in C57BL/6J mice by altering the intestinal mi-crobiota associated with microglial activation and BDNF expression[J].Food&Function,2020,11(1):378-391.
[14]LI W,ALI T,HE K,et al.Ibrutinib alleviates LPS-in-duced neuroinflammation and synaptic defects in a mouse model of depression[J].Brain,Behavior,and Im-munity,2021,92:10-24.
[15]MKYNARSKA E,GADZINOWSKA J,TOKAREK J,et al.The role of the microbiome-brain-gut axis in the pathogenesis of depressive disorder[J].Nutrients,2022,14(9).
[16]KIECOLT-GLASER J K,WILSON S J,BAILEY M L,et al.Marital distress,depression,and a leaky gut:Translo-cation of bacterial endotoxin as a pathway to inflamma-tion[J].Psychoneuroendocrinology,2018,98:52-60.
[17]LI D,LIU R,WANG M,et al.3β-Hydroxysteroid dehy-drogenase expressed by gut microbes degrades testos-terone and is linked to depression in males[J].Cell Host&Microbe,2022,30(3):329-339.e5.
[18]YIN W,L魻F M,CHEN R,et al.Mediterranean diet and depression:A population-based cohort study[J].Inter-national Journal of Behavioral Nutrition and Physical Activity,2021,18(1):153.
[19]BAYES J,SCHLOSS J,SIBBRITT D.The effect of a Mediterranean diet on the symptoms of depression in young males(the“AMMEND:A Mediterranean Diet in MEN with Depression”study):A randomized controlled trial[J].The American Journal of Clinical Nutrition,2022,116(2):572-580.
[20]BRTTINA H,KIMBER L S.RE:Current WHO recom-mendation to reduce free sugar intake from all sources to below 10%of daily energy intake for supporting overall health is not well supported by available evidence[J].The American Journal of Clinical Nutrition,2022,116(6):1904.
[21]YAO S,ZHANG M,DONG S S,et al.Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression[J].Nature Human Behaviour,2022,6(11):1569-1576.
[22]XU Z,LIU Z X,DONG X G,et al.Fecal microbiota transplantation from healthy donors reduced alcohol-in-duced anxiety and depression in an animal model of chronic alcohol exposure[J].Chinese Journal Physiology,2018,61(6):360-371.
[23]RAO J,XIE R,LIN L,et al.Fecal microbiota transplan-tation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depres-sive-like behavior[J].The European Journal of Neuro-science,2021,53(11):3598-3611.
[24]LAM S,BAI X,SHKOPOROV A N,et al.Roles of the gut virome and mycobiome in faecal microbiota trans-plantation[J].The Lancet Gastroenterology&Hepatolo-gy,2022,7(5):472-484.
[25]KUROKAWA S,KISHIMOTO T,MIZUNO S,et al.The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome,functional diarrhea and functional constipation:An open-label observational study[J].Journal of Affective Disorders,2018,235:506-512.
[26]LIN H,GUO Q,WEN Z,et al.The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome(IBS-D)patients with anxiety and depression behaviors[J].Microbial Cell Factories,2021,20(1):233.
[27]DOLL J P K,V魣ZQUEZ-CASTELLANOS J F,SCHAUB A C,et al.Fecal microbiota transplantation(FMT)as an adjunctive therapy for depression-case re-port[J].Front Psychiatry,2022,13:815422.
[28]KAZEMI A,NOORBALA A A,AZAM K,et al.Effect of probiotic and prebiotic vs placebo on psychological out-comes in patients with major depressive disorder:A ran-domized clinical trial[J].Clinical Nutrition,2019,38(2):522-528.
[29]BUROKAS A,ARBOLEYA S,MOLONEY R D,et al.Targeting the microbiota-gut-brain axis:Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice[J].Biological Psychia-try,2017,82(7):472-487.
[30]TARUTANI S,OMORI M,IDO Y,et al.Effects of 4G-beta-D-Galactosylsucrose in patients with depres-sion:A randomized,double-blinded,placebo-controlled,parallel-group comparative study[J].Journal of Psychi-atric Research,2022,148:110-120.
[31]HADI A,SEPANDI M,MARX W,et al.Clinical and psychological responses to synbiotic supplementation in obese or overweight adults:A randomized clinical trial[J].Complementary Therapies in Medicine,2019,47:102216.
[32]HAGHIGHAT N,RAJABI S,MOHAMMADSHAHI M.Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level,depression and anxiety symptoms in hemodialysis patients:A random-ized,double-blinded,clinical trial[J].Nuritional Neuro-science,2021,24(6):490-499.
[33]HAGHIGHAT N,MOHAMMADSHAHI M,SHAYAN-POUR S,et al.The effect of synbiotic and probiotic sup-plementation on mental health parameters in patientsun-dergoing hemodialysis:A double-blind,randomized,placebo-controlled trial[J].Indian Journal of Nephrolo-gy,2021,31(2):149-156.
[34]CRISTIANO C,CUOZZO M,CORETTI L,et al.Oral sodium butyrate supplementation ameliorates paclitax-el-induced behavioral and intestinal dysfunction[J].Biomedicine&Pharmacotherapy,2022,153:113528.
[35]BATTLE D E.Diagnostic and statistical manual of men-tal disorders(DSM)[J].Codas,2013,25(2):191-192.
[36]MAENNER M J,WARREN Z,WILLIAMS A R,et al.Prevalence and characteristics of autism spectrum disor-der among children aged 8 years-autism and develop-mental disabilities monitoring network,11 sites,united states,2020[J].Morbidity and Mortality Weekly Report,Surveillance Summaries,2023,72(2):1-14.
[37]TAYLOR M J,ROSENQVIST M A,LARSSON H,et al.Etiology of autism spectrum disorders and autistic traits over time[J].JAMA Psychiatry,2020,77(9):936-943.
[38]WANG J,MA B,WANG J,et al.Global prevalence of autism spectrum disorder and its gastrointestinal symp-toms:A systematic review and meta-analysis[J].Front Psychiatry,2022,13:963102.
[39]IGLESIAS-V魣ZQUEZ L,VAN GINKEL RIBA G,ARI-JA V,et al.Composition of gut microbiota in children with autism spectrum disorder:A systematic review and meta-analysis[J].Nutrients,2020,12(3).
[40]KANDEEL W A,MEGUID N A,BJ?RKLUND G,et al. Impact of clostridium bacteria in children with autism spectrum disorder and their anthropometric measure-ments[J].Journal of Molecular Neuroscience:MN,2020,70(6):897-907.
[41]ADAMS J B,JOHANSEN L J,POWELL L D,et al. Gastrointestinal flora and gastrointestinal status in chil-dren with autism—comparisons to typical children and correlation with autism severity[J].BMC Gastroenterolo-gy,2011,11:22.
[42]FINEGOLD S M,MOLITORIS D,SONG Y,et al.Gas- trointestinal microflora studies in late-onset autism[J].Clinical Infectious Diseases,2002,35(1):S6-s16.
[43]NEEDHAM B D,ADAME M D,SERENA G,et al. Plasma and fecal metabolite profiles in autism spectrum disorder[J].Biological Psychiatry,2021,89(5):451-462.
[44]KANG D W,PARK J G,ILHAN Z E,et al.Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children[J].PLoS One,2013,8(7):e68322.
[45]WANG L,CHRISTOPHERSEN C T,SORICH M J,et al.Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp.in feces of children with autism[J].Applied and Environ-mental Microbiology,2011,77(18):6718-6721.
[46]DE ANGELIS M,PICCOLO M,VANNINI L,et al.Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified[J].PLoS One,2013,8(10):e76993.
[47]AVERINA O V,KOVTUN A S,POLYAKOVA S I,et al. The bacterial neurometabolic signature of the gut micro-biota of young children with autism spectrum disorders[J].Journal of Medical Microbiology,2020,69(4):558-571.
[48]TOMOVA A,HUSAROVA V,LAKATOSOVA S,et al. Gastrointestinal microbiota in children with autism in Slovakia[J].Physiology&Behavior,2015,138:179-87.
[49]SANTOCCHI E,GUIDUCCI L,FULCERI F,et al.Gut to brain interaction in Autism Spectrum Disorders:a randomized controlled trial on the role of probiotics on clinical,biochemical and neurophysiological parameters[J].BMC Psychiatry,2016,16:183.
[50]MACFABE F D.Short-chain fatty acid fermentation products of the gut microbiome:Implications in autism spectrum disorders[J].Microbial Ecology in Health and Disease,2012,23(1):1-24.
[51]WANG L,CHRISTOPHERSEN C T,SORICH M J,et al.Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder[J].Digestive Diseases and Sciences,2012,57(8):2096-2102.
[52]BREIT S,KUPFERBERG A,ROGLER G,et al.Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders[J].Front Psychiatry,2018,9:44.
[53]HUANG F,WU X.Brain neurotransmitter modulation by gut microbiota in anxiety and depression[J].Frontiers in Cell and Developmental Biology,2021,9:649103.
[54]MA Q,XING C,LONG W,et al.Impact of microbiota on central nervous system and neurological diseases:The gut-brain axis[J].Journal of Neuroinflammation,2019,16(1):53.
[55]YAGHOUBFAR R,BEHROUZI A,ASHRAFIAN F,et al.Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice[J].Scientific Reports,2020,10(1):22119.
[56]LEES H J,SWANN J R,WILSON I D,et al.Hippurate:The natural history of a mammalian-microbial cometabo-lite[J].Journal ofProteome Research,2013,12(4):1527-46.
[57]NAVARRO F,LIU Y,RHOADS J M.Can probiotics benefit children with autism spectrum disorders?[J].World Journal of Gastroenterology,2016,22(46):10093-10102.
[58]DINAN T G,CRYAN J F.Gut instincts:Microbiota as a key regulator of brain development,ageing and neu-rodegeneration[J].Journal of Physiology,2017,595(2):489-503.
[59]BUFFINGTON S A,DI PRISCO G V,AUCHTUNG T A,et al.Microbial reconstitution reverses maternal diet-In-duced social and synaptic deficits in offspring[J].Cell,2016,165(7):1762-1775.
[60]CARVALHO A F,FIRTH J,VIETA E.Bipolar disorder reply[J].Nature English Journal of Medicine,2020,383 (14):1398.
[61]ANDERSON G,MAES M.Bipolar disorder:Role of im-mune-inflammatory cytokines,oxidative and nitrosative stress and tryptophan catabolites[J].Current Psychiatry Reports,2015,17(2):8.
[62]MCINTYRE R S,SUBRAMANIAPILLAI M,SHEKOTIKHINA M,et al.Characterizing the gut micro-biota in adults with bipolar disorder:A pilot study[J].Nutritional Neuroscience,2021,24(3):173-180.
[63]LAI J,JIANG J,ZHANG P,et al.Gut microbial clues to bipolar disorder:State-of-the-art review of current find-ings and future directions[J].Clinic Translation Medicine,2020,10(4):e146.
[64]LAI J,ZHANG P,JIANG J,et al.New evidence of gut microbiota involvement in the neuropathogenesis of bipo-lar depression by TRANK1 modulation:Joint clinical and animal data[J].Frontiers in Immunology,2021,12:789647.
[65]RONG H,XIE X H,ZHAO J,et al.Similarly in depres-sion,nuances of gut microbiota:Evidences from a shot-gun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major de-pressive episode patients[J].Journal of Psychiatric Re-search,2019,113:90-99.
[66]SLEIMAN S F,HENRY J,AL-HADDAD R,et al.Ex-ercise promotes the expression of brain derived neu-rotrophic factor(BDNF)through the action of the ketone bodyβ-hydroxybutyrate[J].Elife,2016,5.
[67]DICKERSON F,ADAMOS M,KATSAFANAS E,et al.Adjunctive probiotic microorganisms to prevent rehospi-talization in patients with acute mania:A randomized controlled trial[J].Bipolar Disorders,2018,20(7):614-621.
[68]REININGHAUS E Z,WETZLMAIR L C,FELLENDORF F T,et al.The impact of probiotic supplements on cog-nitive parameters in euthymic individuals with bipolar disorder:A pilot study[J].Neuropsychobiology,2018:1-8.
[69]GALLUCCI A,PATTERSON K C,WEIT A R,et al.Microbial community changes in a female rat model of Rett syndrome[J].Progress in Neuropsychopharmacology&Biological Psychiatry,2021,109:110259.
[70]STRATI F,CAVALIERI D,ALBANESE D,et al.Al-tered gut microbiota in Rett syndrome[J].Microbiome,2016,4(1):41.
[71]NEIER K,GRANT T E,PALMER R L,et al.Sex dis-parate gut microbiome and metabolome perturbations precede disease progression in a mouse model of Rett syndrome[J].Communications Biology,2021,4(1):1408.
[72]THAPA S,VENKATACHALAM A,KHAN N,et al.As-sessment of the gut bacterial microbiome and metabolome of girls and women with Rett Syndrome[J].PLoS One,2021,16(5):e0251231.
[73]GAN Y,CHEN Y,ZHONG H,et al.Gut microbes in central nervous system development and related disorders[J].Frontiers in Immunology,2023,14:1288256.
[74]KHOURY E S,SHARMA A,RAMIREDDY R R,et al.Dendrimer-conjugated glutaminase inhibitor selectively targets microglial glutaminase in a mouse model of Rett syndrome[J].Theranostics,2020,10(13):5736-5748.
[75]MAEZAWA I,JIN L W.Rett syndrome microglia dam-age dendrites and synapses by the elevated release of glutamate[J].Journal of Neuroscience,2010,30(15):5346-56.
[76]EICHER T P,MOHAJERI M H.Overlapping mecha-nisms of action of brain-active bacteria and bacterial metabolites in the pathogenesis of common brain diseases[J].Nutrients,2022,14(13).
[77]ZHENG P,ZENG B,LIU M,et al.The gut microbiome from patients with schizophrenia modulates the gluta-mate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice[J].Science Advanture,2019,5(2):eaau8317.
[78]SAYAL K,PRASAD V,DALEY D,et al.ADHD in children and young people:prevalence,care pathways,and service provision[J].Lancet Psychiatry,2018,5(2):175-186.
[79]BONVICINI C,CORTESE S,MAJ C,et al.DRD4 48 bp multiallelic variants as age-population-specific biomark-ers in attention-deficit/hyperactivity disorder[J].Trans-lation Psychiatry,2020,10(1):70.
[80]BOONCHOODUANG N,LOUTHRENOO O,CHATTI-PAKORN N,et al.Possible links between gut-microbiota and attention-deficit/hyperactivity disorders in children and adolescents[J].European Journal of Nutrition,2020,59(8):3391-3403.
[81]BULL-LARSEN S,MOHAJERI H M.The potential influ- ence of the bacterial microbiome on the development and progression of ADHD[J].Nutrients,2019,11(11):2805.
[82]AARTS E,EDERVEEN T H A,NAAIJEN J,et al.Gut microbiome in ADHD and its relation to neural reward anticipation[J].PLoS One,2017,12(9):e0183509.
[83]P魧RTTY A,KALLIOM魧KI M,WACKLIN P,et al.Apossible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood:A randomized trial[J].Pediatric Research,2015,77(6):823-828.
[84]BRAVO J A,FORSYTHE P,CHEW M V,et al.Inges- tion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve[J].Proceedings of the National Acade-my of Sciences of the United States of America,2011,108(38):16050-5.
[85]ANA C,ANTONIO J,ANTONIO M,et al.Current evi-dence on the role of the gut microbiome in ADHD pathophysiology and therapeutic implications[J].Nutri-ents,2021,13(1):249.
[86]DERVOLA K S,ROBERG B A,W諂IEN G,et al.Marine Ο-3 polyunsaturated fatty acids induce sex-specific changes in reinforcer-controlled behaviour and neuro-transmitter metabolism in a spontaneously hypertensive rat model of ADHD[J].Behavior Brain Function,2012,8:56.
[87]NYANGAHU D D,LENNARD K S,BROWN B P,et al.Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity[J].Microbiome,2018,6(1):124.
文章出自SCI论文网转载请注明出处:https://www.lunwensci.com/ligonglunwen/81757.html